Abstract
Hematopoietic stem cells (HSCs) are considered the only cell type capable of self-renewing and generating all blood lineages. HSCs are further assumed to be rare, barely proliferating, and not contributing to short-term hematopoietic recovery after transplantation. Although widely accepted, individual definitions have been challenged. Tracking of murine HSCs showed continuous contribution during steady-state hematopoiesis. Similarly, single HSCs can reconstitute the entire murine host illustrating extensive proliferative capabilities without immediate exhaustion while maintaining long-term persistence.
Studying these findings in humans is challenging. Knowledge about the engraftment kinetics and contribution of human HSCs during recovery is limited to clinical trials of gene therapy patients enrolled in clonal tracking studies using retroviral integration site analysis (ISA). However, the tracking of human HSCs remains under-investigated due to the limited number of specimens possible to obtain, especially during the early phases of reconstitution where blood cell counts are low. In addition, clone tracking is impacted by technical limitations. While ISA is reliable to monitor the potential outgrowth of dominant clones, low sensitivity and high error rates require significant data exclusion and sophisticated statistical tests to ensure data reliability (Adair et al. 2020, Molecular Therapy MCD). Lack of sensitivity and loss of low abundance clones can be overcome by increasing the frequency of sampling (high density) as well as repeated sampling, aspects hard to achieve in patients.
To investigate the clonal dynamics in the early phases of hematopoietic reconstitution and determine the onset of HSC contribution, we performed high-density sampling for ISA in nonhuman primates (NHPs). In the first month of hematopoietic recovery, weekly blood samples were taken to enhance data density. We applied a new clonal tracking approach to reliably detect the contribution of multipotent long-term persisting HSCs during neutrophil recovery even in samples with low cellularity during early recovery. Animals were followed for 4.5 years to collect HSC clone signatures, and multipotent stem cell clones were retrospectively identified in early samples. Finally, observed changes in clonal diversity in vivo were used to inform a simulation of hematopoietic reconstitution to determine the temporal involvement of HSCs.
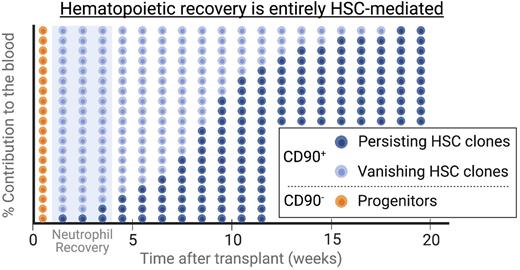
Contribution of multipotent long-term persisting HSCs clones was detected in the very first blood samples taken 2 to 3 weeks post-transplant during neutrophil recovery. HSCs became the dominant source for mature blood cells in the peripheral blood as early as 50 days post-transplant. Based on these finding, simulations predicted that hematopoietic recovery is primarily HSC driven, differentiating HSC clones are the main source of blood production during recovery, and that long-term persisting HSC clones expand forming clonal pools. The ability of HSCs to symmetrically expand was confirmed ex vivo in colony-forming cells assays as well as in vivo using ISA and tracking of expressed barcodes. As predicted by the simulation, we observed a rapid decline of clonal diversity and individual HSCs symmetrically expand forming clonal pools.
Here, we show evidence that long-term persisting multipotent HSCs actively contribute during early hematopoietic reconstitution after transplantation. Enhanced sampling showed that multipotent HSCs produce neutrophils during recovery, are the main source of mature blood cells as early as 50 days post-transplant, and symmetrically divide to form clonal pools long-term. Most importantly, observed changes in the clonal diversity during early recovery suggest a stochastic HSC engraftment and differentiation pattern rather than a bi-phasic reconstitution initially driven by short-term progenitors. Early blood production is driven by these HSC clones that are not predetermined but stochastically contribute to a limited number of lineages before they disappear - a population of cells previously incorrectly identified as lineage-restricted short-term engrafting progenitors. Consequently, we here propose a revised model of hematopoietic reconstitution and provide novel insights into HSC biology with direct implications for the design of HSC transplantation as well as gene therapy studies.
Disclosures
Radtke:Forty Seven: Consultancy; Ensoma: Consultancy. Perez:Umoja Biopharma: Current Employment, Current holder of stock options in a privately-held company. Kiem:Ensoma: Consultancy; VOR Biopharma: Consultancy; Homology Medicines: Consultancy; Rocket Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal